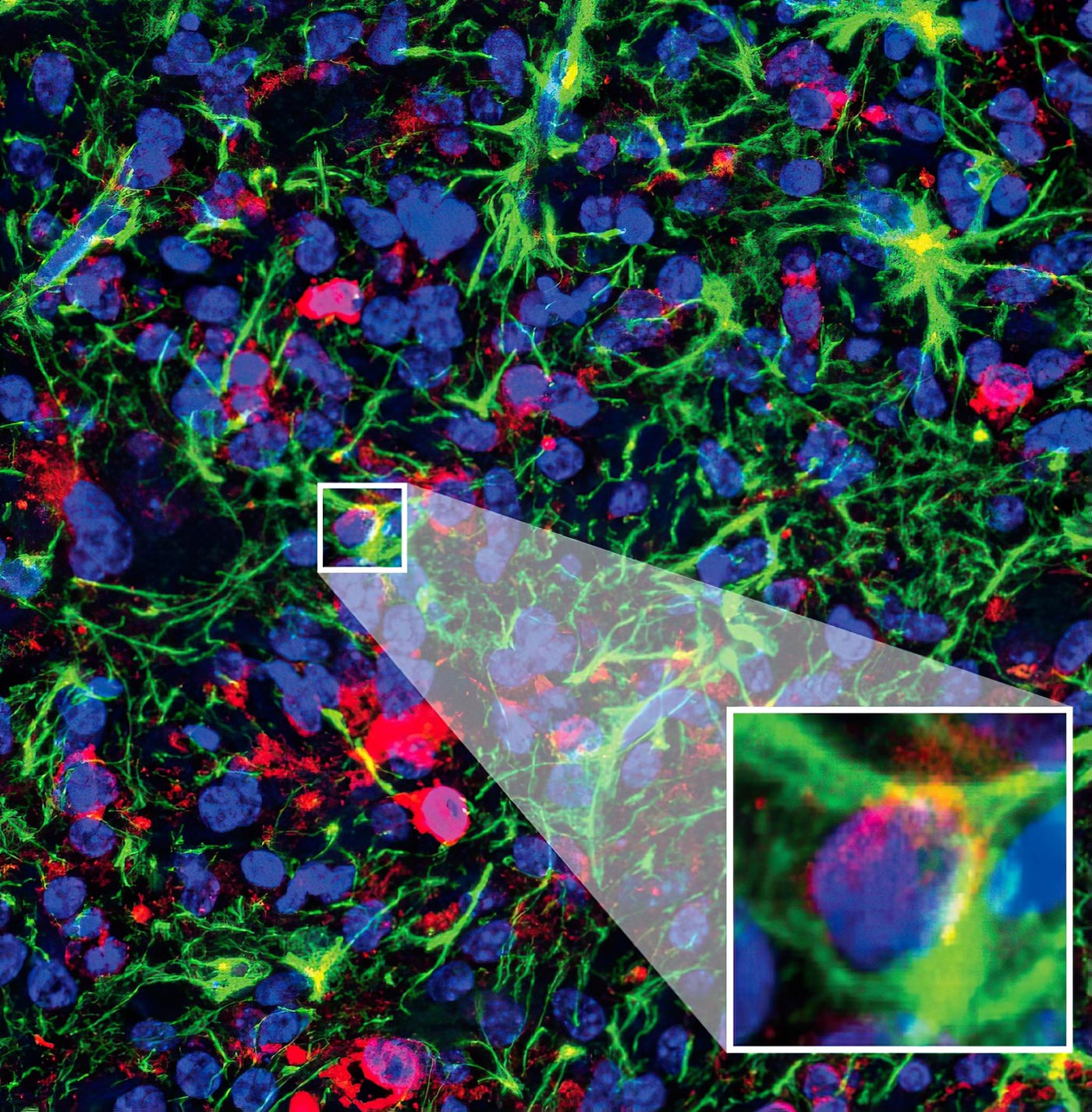
Cold Spring Harbor Laboratory Professor Adrian Krainer developed several antisense oligonucleotide (ASO) molecules for a new potential cancer treatment, but the most potent was ASO5. When mice were treated with ASO5, their tumor cells (stained red) grew slower and began differentiating into healthy cells (stained green). Credit: Krainer lab/Cold Spring Harbor Laboratory
Researchers develop a potential therapeutic for lethal pediatric brain cancer, DIPG, using ASO technology. The treatment slows tumor growth and increases survival rates in mice.
Cold Spring Harbor Laboratory Professor Adrian Krainer, known for his groundbreaking research on antisense oligonucleotides (ASOs) and the development of Spinraza® for spinal muscular atrophy (SMA), has created a potential therapeutic for diffuse intrinsic pontine glioma (DIPG). DIPG is a lethal pediatric brain cancer with limited treatment options. Krainer and his team developed an ASO drug that shuts down the mutated protein H3.3K27M, which slows tumor growth, reverses some cancer cell changes, and increases survival rates in mice. The treatment would likely need to be combined with radiation or immunotherapy before clinical trials can begin. The team is exploring ways to enhance the therapy’s effectiveness.
Diffuse intrinsic pontine glioma (DIPG) is a lethal pediatric brain cancer that often kills within a year of diagnosis. Surgery is almost impossible because of the tumors’ location. Chemotherapy has debilitating side effects. New treatment options are desperately needed.
Cold Spring Harbor Laboratory Professor Adrian Krainer is best known for his groundbreaking research on antisense oligonucleotides (ASOs)—molecules that can control protein levels in cells. His efforts led to Spinraza®the first FDA-approved treatment for a deadly neurodegenerative disease called spinal muscular atrophy (SMA).
Following his success with SMA, Krainer started looking into other diseases where ASOs could make a difference. He soon set his sights on DIPG. “I was contacted by a neurologist and his friend, who had lost her child to DIPG,” Krainer says. “They called to ask if what we did for SMA could be applied. Of course, every disease has its own barriers and obstacles, but it seemed doable. We thought it might be possible to develop a therapy.”
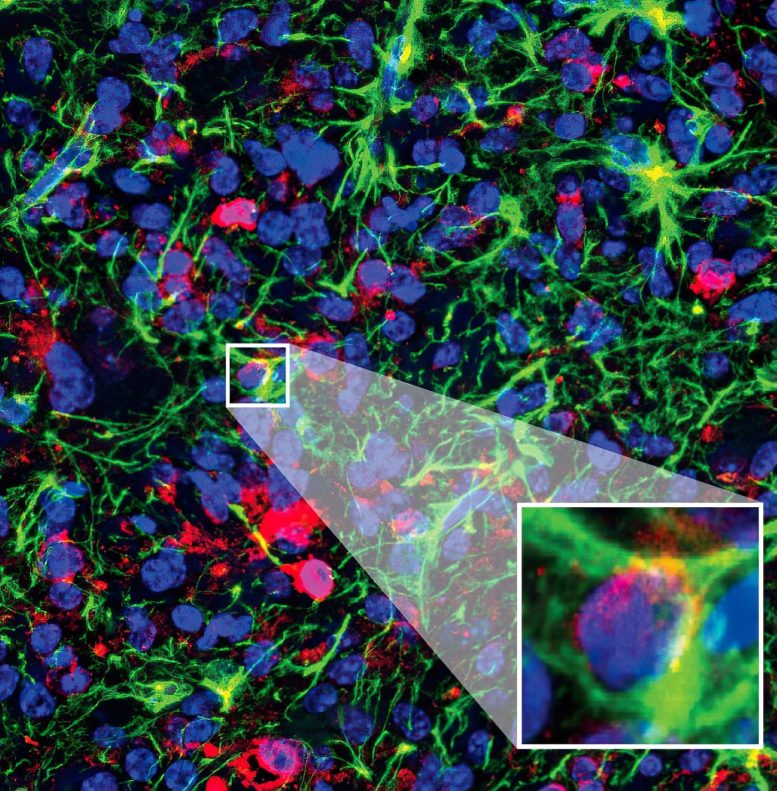
Here we see a close-up of DIPG tumor cells transforming into healthy cells after treatment with the Krainer lab’s new potential ASO drug. Professor Krainer is the deputy director of research at the CSHL Cancer Center. Credit: Krainer lab/Cold Spring Harbor Laboratory
Now, Krainer, graduate student Qian Zhang, and their colleagues have developed a potential therapeutic for DIPG using ASO technology similar to that in Spinraza. This new therapy slowed tumor growth, reversed certain changes in cancer cells, and increased survival rates in mice with DIPG. Krainer’s SMA research laid the foundation for this work.
“While working on Spinraza, we learned how to deliver ASOs to the spinal cord and brain,” he explains. “They have long-lasting effects there. So, we knew there was potential for treating other diseases.”
The new ASO drug works by shutting down a mutated protein called H3.3K27M. In DIPG, the dominant mutation blocks closely related proteins from turning many genes on and off. This leads to uncontrolled cell growth—cancer. When the team used the ASO drug on mice with DIPG, the genes it affected returned to normal. The tumors stopped growing as fast, and the animals lived longer.
“After treatment, the cancer looked very different,” says Krainer. “We could see a lot fewer proliferating cells, and the tumor cells were differentiating into healthy nerve cells. That tells us DIPG’s malignant changes are reversible to an extent.”
While hopeful, Krainer says there is still a long way to go before this new therapeutic can begin clinical trials. Additionally, the potential drug would likely need to be paired with another treatment like radiation or immunotherapy.
“Certainly, we would like this to make it to clinical studies,” he says, “but we didn’t put all our cards into one approach. We’re exploring ways to make this even more effective.”
Reference: “Antisense oligonucleotide therapy for H3.3K27M diffuse midline glioma” 12 April 2023, Science Translational Medicine.
DOI: 10.1126/scitranslmed.add8280
Funding: Cure Starts Now Foundation, Simons Foundation, The V Foundation, St. Giles Foundation, NIH/National Cancer Institute