By University of Texas M. D. Anderson Cancer CenterNovember 10, 2022
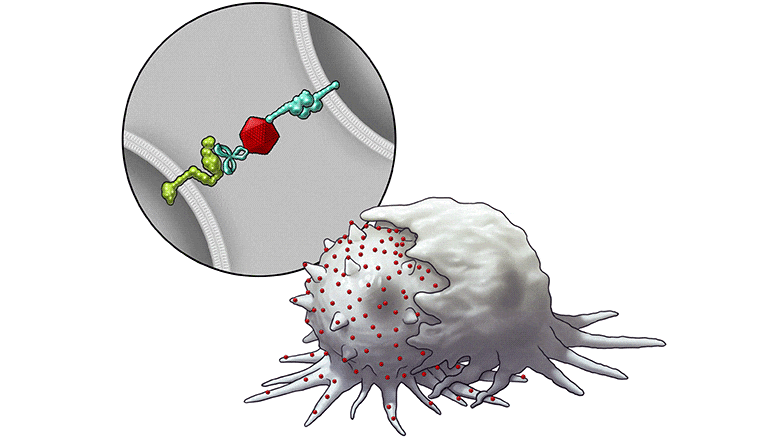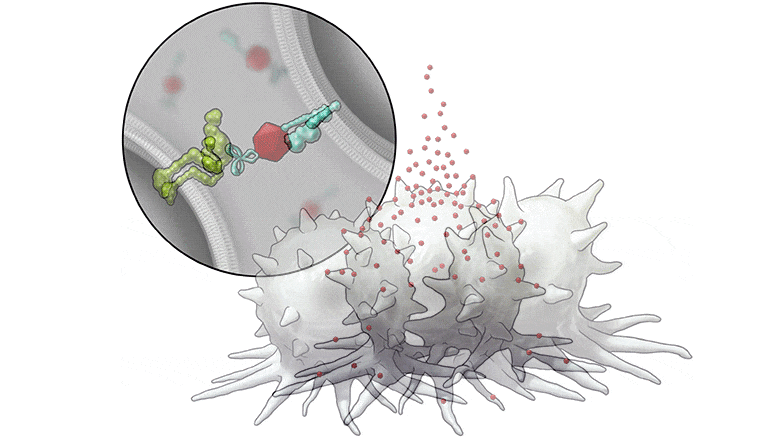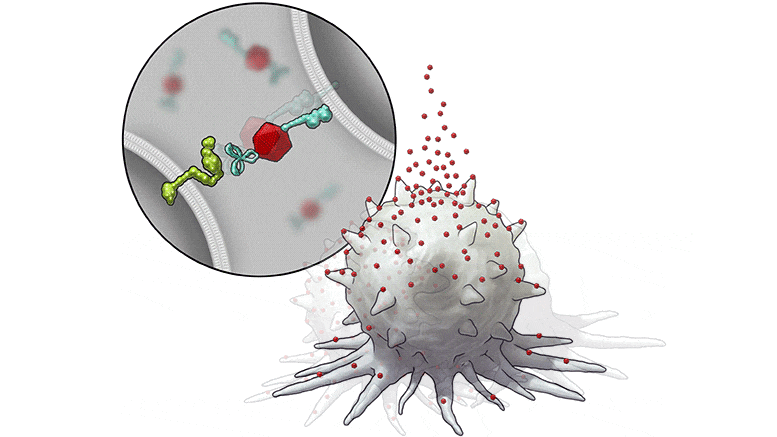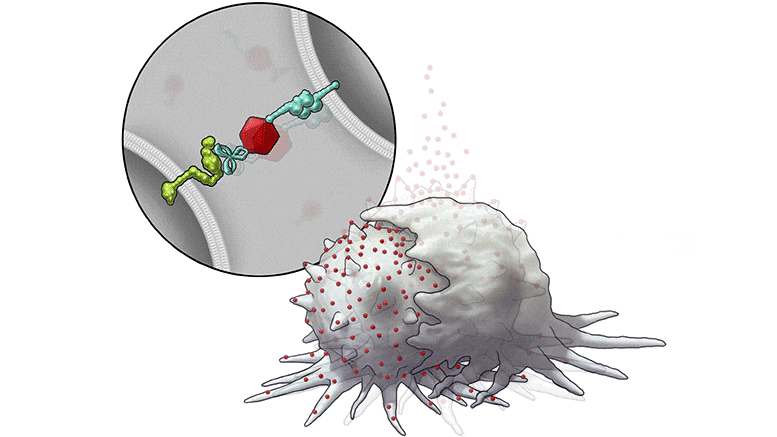
In this series of illustrations, the immune cell does not initially recognize the cancer cell. After BiTN particles (red), which include the “eat me” signal (teal), are attached to the cancer cell, the immune cell recognizes the cell to ingest it. Credit: The University of Texas MD Anderson Cancer Center
Preclinical study uses nanoparticles to attach immune-activating molecules to tumors, sensitizing them to immunotherapy.Scientists have developed a nanotechnology platform that can change the way the immune system sees solid tumor cells, making them more receptive to immunotherapy. This adaptable immune conversion approach has the potential for broad application across many cancer types, according to preclinical findings.The study details the use of this platform to artificially attach an activation molecule to the surface of tumor cells, triggering an immune response in both in vivo and in vitro models. It will be published today (November 10) in the journal Nature Nanotechnology. Wen Jiang, M.D., Ph.D., assistant professor of Radiation Oncology, and Betty Kim, M.D., Ph.D., professor of Neurosurgery, co-led the study, which was conducted by a team of researchers at The University of Texas MD Anderson Cancer Center.“With this new platform, we now have a strategy to convert a solid tumor, at least immunologically, to resemble a hematological tumor, which often has a much higher response rate to immunotherapy treatments,” Jiang said. “If we are able to translate and validate this approach in the clinic, it may enable us to get closer to the maximum level of activity from immunotherapy drugs with cancers that have not traditionally responded well.”
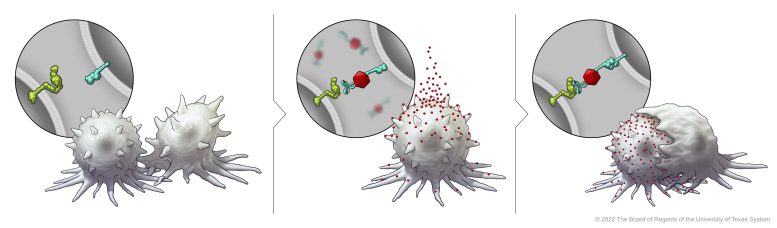
In this illustration, the immune cell does not initially recognize the cancer cell. After BiTN particles (red), which include the “eat me” signal (teal), are attached to the cancer cell, the immune cell recognizes the cell to ingest it. Credit: The University of Texas MD Anderson Cancer Center
Immunotherapy has high response rates in blood cancers like leukemia and lymphoma, but success has been variable across solid tumors. Scientists have been working to further understand the mechanisms prohibiting a better response. One explanation is that varied expression of immune regulatory molecules on blood cancer versus solid tumor cells impacts how they interact with immune cells.The signaling lymphocytic activation molecule family member 7 (SLAMF7) receptor is critical in activating the body’s immune cells against cancer cells, acting as an “eat me” signal. However, it is found almost exclusively on the surface of blood cancer cells and not in solid tumor cells, making it an attractive target for the researchers’ immune conversion approach.To promote the expression of SLAMF7 on solid tumor cells, the researchers developed their bispecific tumor-transforming nanoconjugate (BiTN) platform. These nanosystems are designed with one molecule to bind to the surface of targeted tumor cells and a second molecule to activate an immune response.In this study, the researchers used BiTN with SLAMF7 and a HER2-recognizing antibody to target HER2-positive breast cancer cells. In laboratory models, the nanoconjugate successfully attached SLAMF7 to the breast cancer cells, resulting in phagocytosis, or ingestion, by immune cells. The approach also sensitized the breast cancer cells to treatment with an anti-CD47 antibody, which blocks the “don’t eat me” signal from tumor cells to further increase responses in solid tumors.According to the authors, one of the most exciting things about this platform is its broad potential applications. The approach would not be specific to one cancer type or one regulatory molecule, rather it has the potential to be a universal strategy for several different solid tumor types. As a proof of concept, the authors also developed BiTN with folate instead of the anti-HER2-antibody to target triple-negative breast cancer with similar results.“Because these are engineered constructs, this can be used as a plug-and-play approach to incorporate different tumor-targeting agents or immune molecules onto the surface of the nanoparticle,” Kim said. “For patients with solid tumors that have not responded to immunotherapy, we see this as an added advantage to target the part of the tumor that didn’t respond.”Reference: “Immunological conversion of solid tumours using a bispecific nanobioconjugate for cancer immunotherapy” 10 November 2022, Nature Nanotechnology.
DOI: 10.1038/s41565-022-01245-7The study was supported in part by the Susan G. Komen Foundation Career Catalyst Research Grant, the National Cancer Institute/National Institutes of Health (1K08 CA241070, P30 CA016672), and the United States Department of Defense. A full list of co-authors and disclosures can be found in the full paper.