Credit: Durham University
A team of researchers from Durham University and the University of York has twisted molecules to their limits in order to challenge understanding of chemical bonds.
The scientists examined how much twisting the chemical bonding in an aromatic ring could endure before it broke. They accomplished this by creating overcrowded aromatic rings, utilizing tropylium instead of benzene, which shares electrons around a ring of seven carbon atoms.
Each of these carbon atoms can be functionalized and having seven attachment points in the ring, rather than the six carbon atoms of benzene, allowed the researchers to cram more groups around the edge of the aromatic ring, causing more strain. The researchers found that low levels of overcrowding made the ring twist, but without breaking its aromatic bonding.
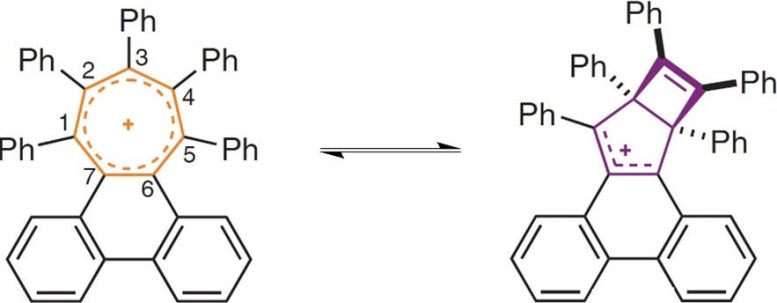
The 7-membered ring (left) becomes so crowded around its periphery that it rearranges by pinching across the middle (right), with the molecule alternating between the two structures. Credit: Durham University
By adding progressively larger groups around the edge of the ring, the team twisted the ring further, eventually causing the aromatic bonding to break.
The electrons no longer circle the seven carbon atoms and instead, the ring pinches across its middle to form two smaller flat rings. Surprisingly, the researchers found there is a balance point, where the ring jumps back and forth between the aromatic structure and the two smaller rings. One molecule made in this study spends 90% of its time as the pinched structure and 10% of its time as a larger aromatic ring.
Full study results have been published in the journalNature Chemistry.
Reflecting on the study results, Dr. Paul McGonigal of the DOI: 10.1038/s41557-023-01149-6
The study was funded by the Engineering and Physical Sciences Research Council (EPSRC).